Menu

Group Leader: Petr Cejka
Researchers:Ananya Acharya, Elda Cannavò Cejka, Ilaria Ceppi, Swagata Halder, Sean Michael Howard, Giordano Reginato, Aurore Sanchez
Deoxyribonucleic acid (DNA) stores genetic information that contains instructions for the proper development and function of all living organisms. The integrity of DNA must be preserved during the life cycle in order to maintain cellular functions and to pass information encoded in it onto the next generation. It has been estimated that each cell in a human body acquires tens of thousands of DNA lesions per day. The sources of DNA damage may stem from the environment, such as sunlight or chemicals, or result from regular cellular processes such as metabolism. These events represent a major challenge: if left unrepaired, the lesions could block access to the genetic information and prevent faithful replication (copying) of the DNA molecule. On the other hand, incorrect repair may lead to mutations (changes in genetic information) or chromosomal aberrations (larger scale rearrangements of genetic material). These events may threaten cell viability or, in some cases, result in uncontrolled cell division (cancer).
Throughout evolution, cells have evolved a number of DNA repair pathways specialized for the various classes of DNA damage. Our interest in these mechanisms is stimulated by the fundamental importance these processes play in life. Many DNA repair factors are essential for viability – cells cannot exist without them. Others are important only in special cases – hereditary or sporadic defects in some components of the repair machinery lead to a variety of syndromes characterized by premature aging, cancer predisposition or other abnormalities. Finally, the efficiency of DNA repair mechanisms often affects cancer chemotherapy: a number of drugs that are being used to treat cancer act by causing DNA damage. The mechanisms of action of these drugs as well as repair strategies are often not well understood.
Our research group is interested in DNA repair mechanisms from a basic research standpoint: we want to learn how these pathways operate in healthy cells and how defects lead to abnormalities and disease. Specifically, we focus on a DNA repair pathway termed homologous recombination. Homologous recombination is a highly intricate complex of processes, which repairs breaks in DNA strands. Most cells contain more than one copy of genetic information in each cell, and homologous recombination can exploit that in a very elegant manner. It can restore the integrity of the damaged DNA molecule by using genetic information stored in the identical (or homologous) copy of DNA. This process may thus restore DNA integrity in a largely accurate manner. Homologous recombination is highly conserved in evolution: the mechanism in the bacterium Escherichia coli or in the yeast Saccharomyces cerevisiae is very similar to the mechanism in human cells. This observation underlines the fundamental importance of this pathway in all kingdoms of life. Also, by using the simple organisms as research models, we can learn about homologous recombination in an experimentally more feasible setup. Our research group is using both Saccharomyces cerevisiae and human systems.
Currently, we are using mostly biochemical methods to answer fundamental questions in biology. We express and purify recombinant proteins, assemble multiprotein complexes, and analyze their behavior on synthetic structures that mimic their physiological substrates. Genetic and cell biological techniques are suitable to identify components of new or existing pathways and phenotypic analysis often infers a specific function, yet it becomes limiting when trying to elucidate detailed molecular mechanisms. Redundant and overlapping pathways often further complicate interpretation of in-vivo based experiments. Biochemical analysis is in contrast very powerful to explain underlying molecular mechanisms.
Projects
Group leaders: Petr Cejka
Researchers: Elda Cannavò Cejka, Scientist / Sean Michael Howard, Scientist / Maryna Levikova, University of Zurich / Cosimo Pinto, University of Zurich
Status: In progress
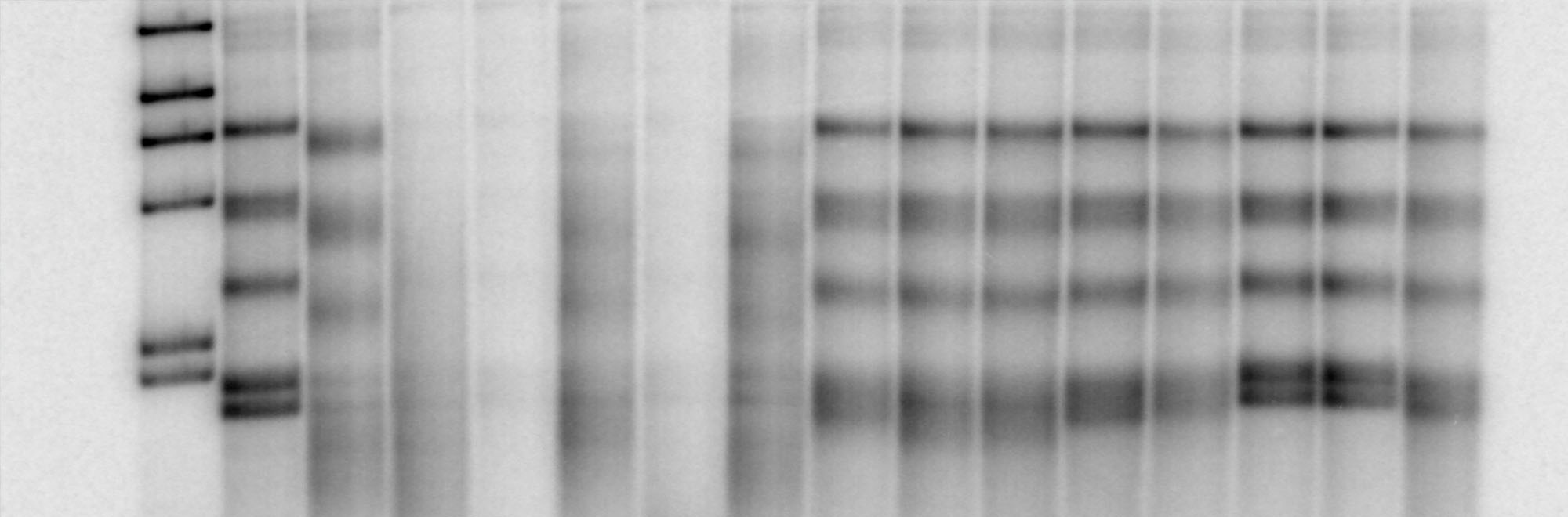
Homologous recombination is initiated by the nucleolytic degradation (resection) of the 5′-terminated DNA strand of the DNA break. This leads to the formation of 3′-tailed DNA, which becomes a substrate for the strand exchange protein RAD51 and primes DNA synthesis during the downstream events in the recombination pathway. DNA end resection thus represents a key process that commits the repair of DNA breaks into recombination. Research from multiple laboratories established that DNA end resection is in most cases a two-step process. It is initiated by the nucleolytic degradation of DNA that is at first limited to the vicinity of the broken DNA end. This is carried out by the Mre11-Rad50-Xrs2 (MRX) complex and Sae2 proteins in yeast, and MRE11-RAD50-NBS1 (MRN) and CtIP proteins in human cells. We could reconstitute these reactions in vitro, and demonstrated that Sae2 and CtIP stimulate a cryptic endonuclease activity within the yeast MRX or human MRN complex, respectively. The activity of Sae2/CtIP is absolutely dependent on its phosphorylation. The reconstituted DNA clipping reaction allows us to investigate the mechanism of this process as well as its regulation by posttranslational modifications and additional protein co-factors.
Downstream of MRX-Sae2 and MRN-CtIP, which process only a limited length of DNA, DNA end resection is further catalyzed by Sgs1-Dna2 or Exo1 in yeast and BLM-DNA2, WRN-DNA2 or EXO1 in human cells. We are interested how the functions of these factors integrate in protein complexes to form molecular machines that are uniquely capable to resect long lengths of DNA, which is required for homologous recombination. We are specifically interested in the Dna2 enzyme, and could show that both yeast Dna2 and human DNA2 possess a cryptic helicase activity. We now investigate how the motor activity of Dna2 promotes DNA end resection, as well as how it is regulated in cells. Finally, as some of these enzymes are upregulated in various human cancers, we are also searching for small molecules capable to inhibit these pathways.
Group leader: Petr Cejka
Status: In progress
Promotion of genetic diversity is a key function of sexual reproduction. At the molecular level, this is controlled by the homologous recombination machinery, which exchanges (recombines) DNA fragments between the maternal and paternal genomes. During this process, joint molecules form between the ‘mum’ and ‘dad’ chromosomes, leading to intermediates termed double Holliday junctions. These joint molecules are then processed in a way that results in the physical exchange of genetic information between the two recombining chromosomes. This so‐called crossover is an integral and essential part of the meiotic cell division. Results from genetic, cell biological and cytological experiments identified the Mlh1‐Mlh3 heterodimer as part of a protein complex that is required for the generation of crossovers during meiotic homologous recombination. However, the mechanism of this reaction is completely unknown. The aim of our research is to analyze the behavior of the purified recombinant Mlh1‐Mlh3 complex as well that of its partners in the processing of double Holliday junctions. We want to show how Mlh1‐Mlh3 can cleave these structures into exclusively crossover recombination products, and therefore explain the molecular mechanism underlying the generation of diversity in meiosis.
So far, we successfully expressed and purified the yeast Mlh1-Mlh3 and human MLH1-MLH3 recombinant proteins into near homogeneity. We could show that the recombinant MutLγ is indeed a nuclease that nicks double‐stranded DNA in the presence of manganese, similarly to the mismatch repair specific MutLα nuclease. MutLγ binds DNA with a high affinity, and shows a marked preference for Holliday junctions, in agreement with its anticipated activity in their processing. Specific DNA recognition has never been observed with any other eukaryotic MutL homologue. Mismatch repair specific MutLα shows no binding preference to mismatched DNA. MutLγ thus represents a new paradigm for the function of the eukaryotic MutL protein family. Unfortunately, to date, we have not seen any activity on joint molecule intermediates (such as Holliday junctions) in the presence of physiological manganese metal cofactor. This will likely require interplay of Mlh1-Mlh3 with other cellular factors (such as Exo1, Msh4-Msh5, etc.), and is the subject of vigorous research in the laboratory at present.
Group leader: Petr Cejka
Status: In progress
n addition to repair double-stranded DNA breaks, homologous recombination helps to stabilize or restart replication forks in the presence of single-stranded DNA breaks or replication-blocking lesions. This likely represents the most important function of recombination, as recombination-deficient human cells can undergo only a very limited number of rounds of DNA replication. The link between stalled or collapsed replication forks and recombination is not understood. It has been inferred that the human MMS22L-TONSL complex might function in this process, but the underlying mechanism is unclear. We could show that MMS22L-TONSL binds RPA-coated single-stranded DNA, which may help recruit the complex to sites of DNA damage. By a direct interaction with the strand exchange protein RAD51, MMS22L-TONSL promotes DNA strand exchange by limiting the assembly of RAD51 on double-stranded DNA. The activity of MMS22L-TONSL then promotes replication fork reversal to protect stalled or stressed replication forks. We will further investigate how MMS22L-TONSL functions alongside other recombination mediators including BRCA2 or the RAD51 paralogs.
Replication fork repair by recombination must be tightly regulated so that it is only activated when needed. Unscheduled DNA recombination might lead to sister chromatid exchanges, loss of heterozygocity, genome rearrangements and other abnormalities, and must be thus tightly controlled. The ultimate goal of our experiments is to understand how MMS22L-TONSL regulates recombination specifically upon replication fork stalling. Our research is anticipated to shed light on the link between DNA replication and repair.

Via Vincenzo Vela 6
6500 Bellinzona, Switzerland
Tel. +41 91 820 0300
Fax +41 91 820 0302
Facebook / Twitter
