Menu

Group Leader: Federica Sallusto
Researchers: Camilla Basso, Antonino Cassotta, Greta Durini, Mengyun Hu, Wenjie Jin, Sandra Jovic, Federico Mele, Samuele Notarbartolo, Luana Perlini, Laura Terzaghi, Daniela Vaqueirinho
The focus of our laboratory is the analysis of the immune response in humans using novel high throughput cell-based assays complemented with powerful analytical technologies, such as next generation sequencing, single cell transcriptomics, metabolomics and proteomics. With our studies, we are defining the signals through which cells of the innate immune system, such as dendritic cells and monocytes, determine the differentiation, proliferation and long-term survival of cells of the adaptive immune system. These studies aim to address fundamental questions related to how the immune system can protect us against different classes of microbial pathogens, such as viruses, or bacteria, and are expected to provide relevant information for the design of new and more effective vaccine strategies. We are also characterizing human T cells that are induced by commensal microbes to define their functional properties and pattern of reactivity in the steady state and in inflammatory conditions. By applying the same experimental approaches, we conduct studies to understand why in patients with chronic or disseminated infections, including children with rare primary immunodeficiencies caused by genetic disorders, the immune system fails to protect the host and how not harmful environmental antigens or self-antigens can cause pathology (allergy and autoimmunity) in some individuals. Recently, we have identified conditions for efficient gene knock in human T cells using the CRISPR/Cas9 system. The system has great potential not only to define physiological mechanisms of T cell activation, differentiation and plasticity, but also to find ways to engineer more effective T cells for immunotherapy.
Projects
Research area: Cellular Immunology
Group leaders: Federica Sallusto
Researchers: Laurent Perez, Scientist, Responsible for the protein production Facility
Status: In progress
From the immune system point of view, microbes (pathogens or commensals) are complex antigens that occupy distinct niches and consequently trigger different types of immune responses. The complexity of the microbial proteome, in particular that of bacteria and fungi, represents a considerable challenge to our capacity to analyse the human T cell response. We are using complementary approaches in order to study the human T cell response to Candida albicans and to identify immunodominant and protective antigens. On the one hand, we are performing a wide screening of HLA-binding peptides, identified through bioinformatic analysis, from 80 fungal proteins belonging to different classes for their capacity to be recognized by different memory T cell subsets. On the other hand, we isolate and identify proteins contained in cell wall extracts that are recognized by human IL-17-producing memory T cells (Th17) and that induce strong immune responses when used as vaccines in mice. These studies are expected to improve our understanding of the immune response to complex pathogens, to define the correlation between class of antigens and type of T cell response elicited, and, finally, to provide useful information for the design of subunit vaccines against C. albicans.
This work is done in collaboration with Alessandro Sette, La Jolla Institute for Allergy and Immunology, La Jolla, CA (US) and Nico Callewaert, Ghent University (BE).
Research area: Cellular Immunology
Group leaders: Federica Sallusto
Status: In progress
Curing cancer has been the major goal of biomedical research in the last decade. Whereas some cancer types like chronic myeloid leukaemia are relatively easy to treat nowadays, others like melanoma or pancreatic adenocarcinoma are still related to a very poor prognosis. Recent studies on immunotherapy showed promising results by exploiting the patient’s immune system to selectively eradicate tumour tissue. However, to make these vaccination strategies more efficient, it is of great importance to find antigens that are highly selective for tumour cells but also easily recognized by the T cells of the patient. So far there are only few studies published that systematically analyse the ability of T cells to recognise known tumour antigens. Most of these studies focus only on effector or memory T cells. Because of this, these studies fail to determine the whole potential of antigen recognition by the T cell repertoire, which includes naïve T cells present only in healthy individuals. However, this repertoire is of great interest for further vaccination strategies, since it may largely determines the efficacy of the included immune response. The aim of this project is, hence, to define the naïve T cell repertoire against a collection of common tumour antigens in healthy donors as well as cancer patients before and after immunotherapy. The results of this study may impact on design of immunotherapies and help to improve the efficacy of this third line of cancer treatment.
Research area: Cellular Immunology
Group leaders: Federica Sallusto
Status: In progress
Allergic sensitization occurs early in childhood upon allergen encounter in persons who have an inherited atopic predisposition. Environmental factors, route, period, and dose of allergen encountered influence the development of the allergic immune response. In the case of allergic individuals, T cell responses show a preferential Th2 phenotype that leads to the production of IgE antibodies, while non-allergic individuals respond to allergens with IgG production and a balanced Th1/Th2 phenotype. IgE is the least abundant class of immunoglobulins but can elicit immediate and strong inflammation through activation of mast cells and basophils via the high affinity receptor FcεRI. We are revisiting the response studying the dynamics of antigen responding CD4 T cells upon natural exposure to allergens in allergic and non-allergic donors, both in and out of allergy season. We are using novel high through put cellular screening approaches to dissect in great detail the phenotype and function of T cells responding to seasonal allergens, like ragweed and timothy grass, or to perennial allergens, such as house dust mite. We are comparing allergic to non-allergic donors to define frequency, class, and distribution in different subsets of the allergen-specific T cells. Since both phenotype and function are related to the location of the antigen challenge, the type and strength of costimulation and cytokines seen during T cell priming, this project will give us insights into the process of sensitization and clinical manifestations which are associated with different types of allergens.
Research area: Cellular Immunology Group leaders: Federica Sallusto Researchers: Federico Mele, Research Assistant Status: In progress
While several studies have reported the identification of Mycobacterium tuberculosis (MTB) antigens, from abundant or easily purified proteins, a truly genome-wide study to identify antigens is lacking. Another unresolved issue relating to MTB immunity is whether different classes of antigens elicit responses that have the same or diverse functional characteristics. MTB antigens described so far are predominantly secreted MTB proteins, some of which are not essential for bacterial survival. As a result, it was hypothesized that secreted proteins might act as decoy antigens, diverting the immune response from recognizing more relevant MTB proteins. In this study we used HLA class II peptide binding predictions, HLA class II multimers, and the screening of T cell libraries, to perform an unbiased, genome-wide analysis of the CD4 T cell response to MTB in latently infected individuals. We showed that human CD4 T cells specific for MTB are highly focused on three broadly immunodominant antigenic islands, all related to bacterial secretion systems, thus refuting the notion that secreted antigens act as a decoy, since both secreted proteins and proteins comprising the secretion system itself are targeted by a fully functional T cell response. In addition, several novel T cell antigens were identified which can be of potential diagnostic use, or as vaccine antigens. Using the T cell library technique we showed also that MTB-responding T cells were highly enriched in libraries derived from the CCR6+CXCR3+ T cell subset (enriched in Th1 cells), and present at lower frequency in libraries from the CCR6+CXCR3– (enriched in Th17 cells) and the CCR6– subset (containing both Th1 and Th2 cells). This pattern of distribution was remarkable consistent: in all 4 donors analyzed more than 80% of the MTB-reactive memory CD4 T cell response resided in the CXCR3+CCR6+ subset. We are currently determining the TCR repertoire diversity of MTB-specific T cells in different memory subsets in by deep sequencing in order to define the origin and lineage relationship of these phenotypically and functionally distinct T cell populations.
This work is shared between IRB and La Jolla Institute for Allergy and Immunology, La Jolla, California (US), and was done together with Cecilia S. Lindestam Arlehamn, Anna Gerasimova, Ryan Henderson,Justine Swann, Jason A. Greenbaum, Yohan Kim, John Sidney, Denise M. McKinney, Howard Grey, Bjoern Peters and Alessandro Sette.
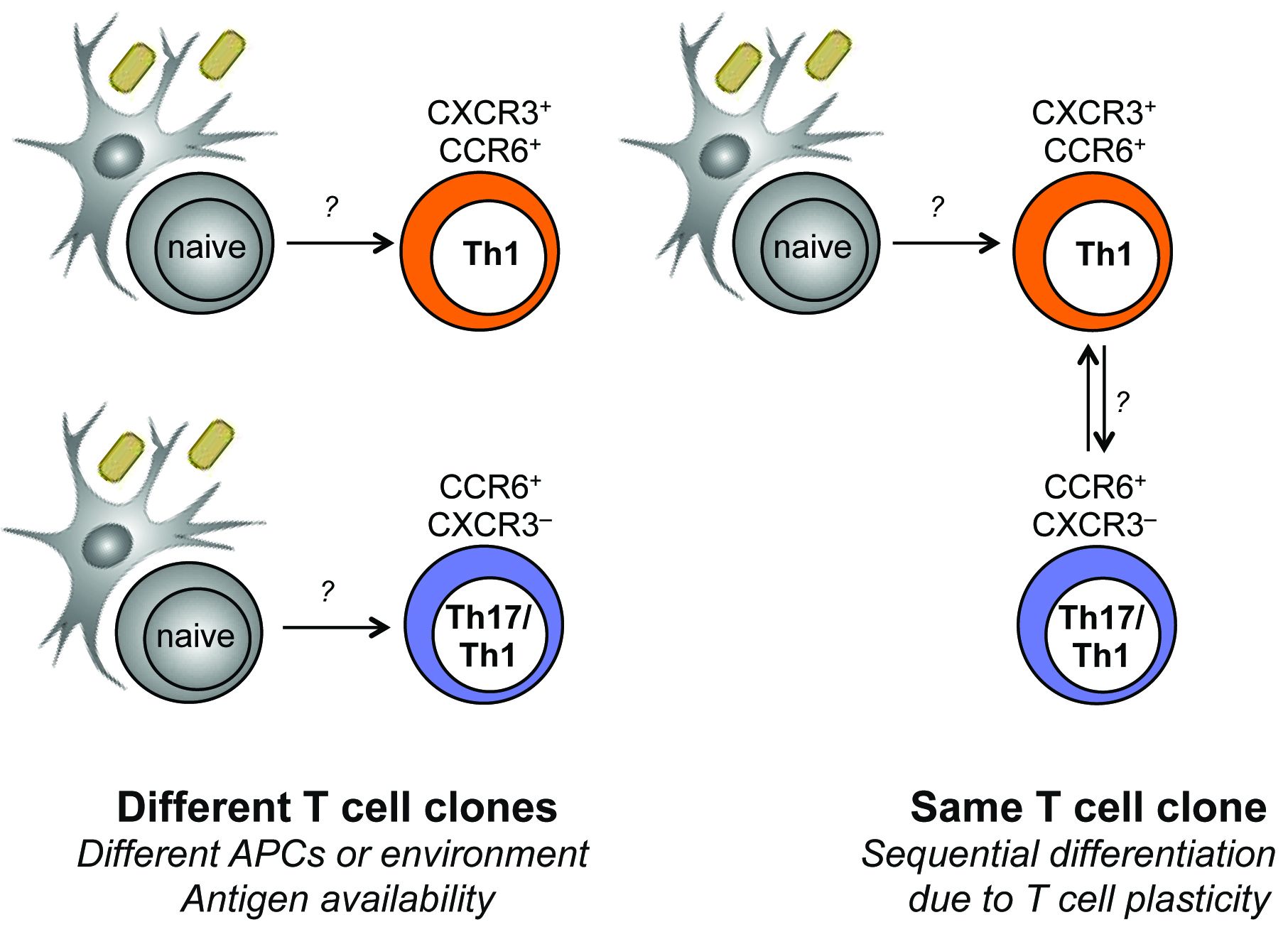
Models for the generation of heterogeneous MTB-responding T cells
Research area: Cellular Immunology
Group leaders: Federica Sallusto
Researchers: Camilla Basso – Scientist, Luana Perlini – Technician
Status: In progress
C. albicans is part of the human commensal flora and poses no risk to healthy individuals. However, under certain circumstances it colonizes the vagina and develops into recurrent infection, affecting 70% of the female population. It is not known why this infection develops and how the immune system can control the pathogen in the vaginal tissue. Earlier studies showed that the IL-17 axis is a crucial part of the host defense mechanism against fungal infections in other tissues. T cells and gd T cells were identified as being the major source of IL-17 in response to C. albicans. By using a mouse model of vaginal candidiasis, we found that protection in the vaginal tissue is not only dependent on T cells but it requires the presence of IL-22-producing innate lymphoid cells as well. Mice either deficient of IL-22 producing T cells (Rag1–/–) or IL-22 producing ILCs (Rorc–/–, Il23a–/–) are unable to control candida infection. Interestingly, mice lacking ILCs are more susceptible and succumb earlier to candida infection than mice lacking only T cells. This observation reveals a so far undescribed interaction between the innate and adaptive arm of the immune system in the vaginal tissue, similar to the one observed in the gut mucosa. ILCs promptly produce IL-22 upon infection and most likely slow down the colonization of the vagina by candida. This first, antigen unspecific wave of immune response is followed by the activation of IL-22 producing T cells which ultimately leads to pathogen clearance.
This work is done in collaboration with Burkhard Becher, University of Zurich (CH).
Research area: Cellular Immunology
Group leaders: Federica Sallusto
Researchers: Camilla Basso – Scientist, Luana Perlini – Technician
Status: In progress
IL-1β is a pleiotropic cytokine that plays a role in several inflammatory disorders in humans and in animal models, including mouse experimental autoimmune encephalomyelitis (EAE). It is produced after cleavage of pro-IL-1β by IL-1 converting enzyme (caspase-1), which in turn is activated by a complex of proteins called inflammasome. IL-1β has been shown to be required for differentiation of human and mouse inflammatory Th17 cells characterized by co-expression of IL-17 and IFN-γ. We found that mice deficient for IL-1β or for a component of the inflammasome (the apoptosis-associated speck-like protein containing a caspase recruitment domain, ASC) did not develop EAE following immunization with myelin oligodendrocyte glycoprotein (MOG) in complete Freund’s adjuvant (CFA) and pertussis toxin (PT). Autoreactive T cells were primed in wild-type (wt), IL-1β–/– and ASC–/– mice. However, while in wt mice T cells proliferated extensively and acquired the capacity to produce inflammatory cytokines, such as IL-17, IL-22, IFN-γ, and GM-CSF, in IL-1β–/– and ASC–/– mice, cells expanded poorly and showed reduced capacity to produce simultaneously inflammatory cytokines, in particular GM-CSF. Interestingly, induction of polyfunctional (IL-17+ IL-22+ IFNγ+ GM-CSF+) T cells in wt mice was dependent on the presence of PT at the time of immunization. PT was found to rapidly induce IL-1β secretion by CD11c+ and Gr1+ myeloid cells, which are highly recruited in secondary lymphoid organs after in vivo PT treatment. Moreover, in mice depleted of Gr1+ myeloid cells, IL-1β production was not induced by PT and priming of polyfunctional T cells was impaired. Taking together, these data support the notion that the disease-inducing effect of PT is due to its ability to induce recruitment of Gr1+ myeloid cells, production of IL-1β, and differentiation of pathogenic polyfunctional T cells.
Research area: Cellular Immunology
Group leaders: Federica Sallusto
Researchers: Federico Mele – Research Assistant, Samuele Notarbartolo – Scientist
Status: In progress
In a previous study, we found that while human resting Th17 clones produced high amounts of IL-17, day 5-activated clones strongly downregulated IL-17 production. At later time points the clones gradually regained the capacity to produce IL-17 as the cells reverted to the resting state. The analysis of transcription factors showed that on day 2 and 5 following restimulation, Th17 clones downregulated RORC mRNA expression. In addition, while both resting and day 5 restimulated Th17 clones phosphorylated STAT3 in response to IL-6, only restimulated clones phosphorylated STAT5 in response to IL-2, consistent with the increased expression of CD25. Overexpression of RORgt significantly restored IL-17 production in activated Th17 clones, and restimulation in the presence of a STAT5 inhibitor rescued RORC mRNA expression and IL-17 production in a proportion of clones. Collectively, these data suggest that in activated human Th17 cells decreased RORgt expression and increased pSTAT5 – which may compete with pSTAT3 for binding to the IL17locus – contribute to the transient downregulation of IL-17 production. Currently, we are extending these studies in two directions. On the one hand we are analyzing Th1 and Th2 clones to understand whether also in these cells cytokine production can be regulated by the activation state of the cell. On the other, we are dissecting the network of signals to find mechanisms of regulation of cytokine gene expression.
Research area: Cellular Immunology Group leaders: Federica Sallusto Status: In progress
Our aim is to develop sensitive methods to study self-reactive T cells in healthy donors and in patients with autoimmune diseases. Since self-reactive T cells may have low avidity and may be present at low frequencies we will combine cell sorting strategies, which highly enrich specific populations, with the T cell library method that allows detection of even low avidity cells. The feasibility of this approach is illustrated by the analysis of T cells from multiple sclerosis (MS) patients that shows that MOG-specific T cells are detectable in most patients and are virtually all present in the CCR6+ memory subset. These findings are consistent with our previous demonstration that CCR6 is required to drive migration of pathogenic T cells in the CNS of mice developing autoimmune encephalomyelitis. We are extending the analysis to other organ-specific autoimmune diseases, in particular pemphigus, for which autoantigens are well characterized, and to other conditions of aberrant responses to self antigens such as Pulmonary alveolar proteinosis, a rare disease characterized by autoantibodies against GM-CSF, and Factor VIII-treated hemofilia in which anti-Factor VIII antibodies develop in response to the therapy.
These studies are done in collaboration with Antonio Uccelli, University of Genova (IT) and Gianna Zambruno, IDI, Rome (IT).
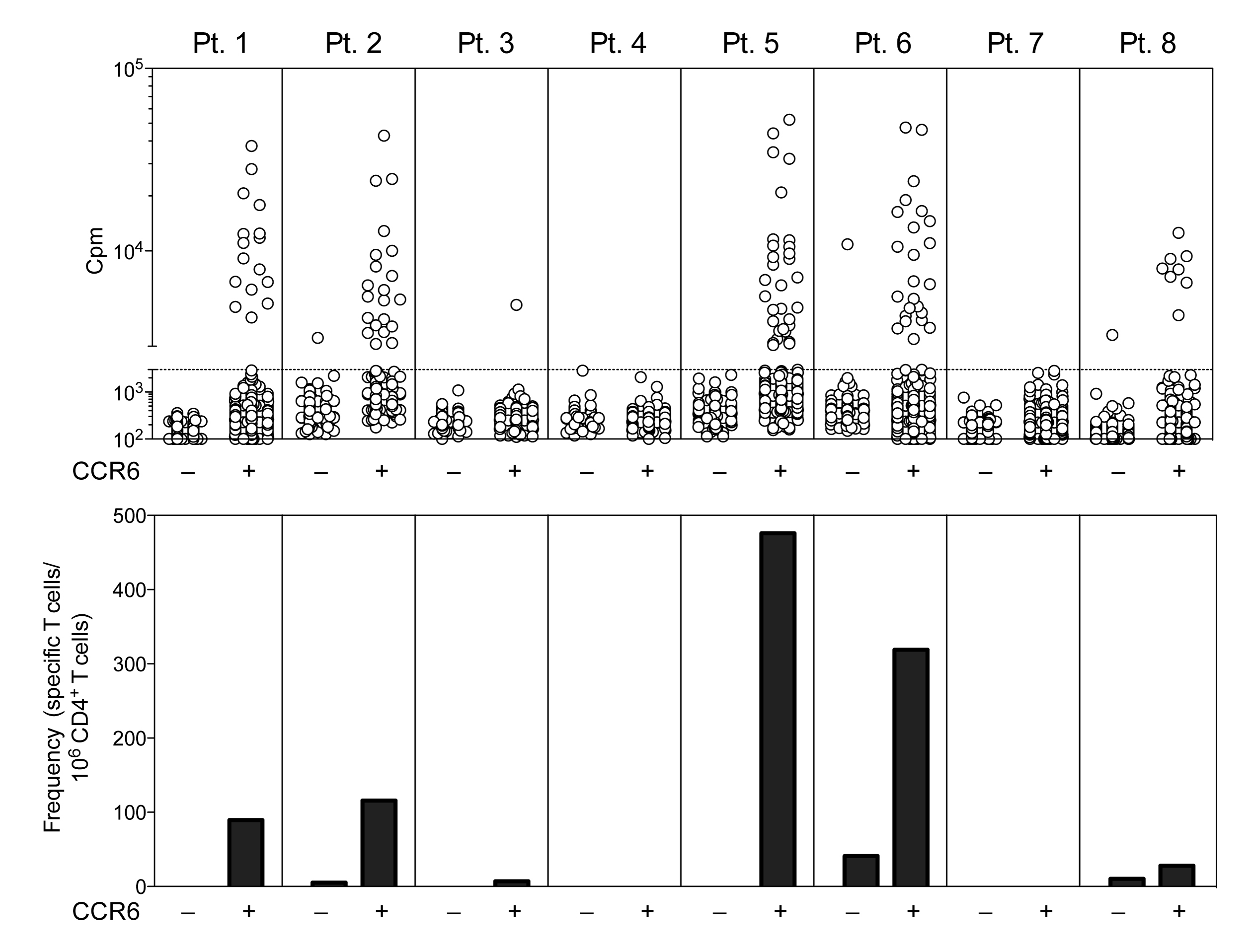
Research area: Cellular Immunology
Group leaders: Federica Sallusto
Researchers: Samuele Notarbartolo, Scientist
Status: In progress
IL-17 producing CD4+ cells (Th17) are a subset of effector T helper cells known to play an important role in host defense against fungi and extracellular bacteria but also involved in tissue inflammation and autoimmune diseases, such as multiple sclerosis, inflammatory bowel disease, and rheumatoid arthritis. The function of Th17 cells depends critically on the range of cytokines produced and on the balance between pro- and anti-inflammatory cytokines. Autoreactive Th17 cells producing IFN-γ and GM-CSF are pathogenic in a mouse model of EAE, while Th17 cells producing IL-10 are not. We have recently shown that C. albicans-specific human Th17 cells produce IL-17 and IFN-γ, while S. aureus-specific human Th17 cells produce IL-17 and, after restimulation, IL-10. While the ontology of the two different Th17 subsets has been clarified, it still remains elusive what is the transcriptional circuit that regulates the expression of the immunoregulatory molecule IL-10. Using a combination of transcriptional profiling and epigenetic approach, we identified the transcription factor c-MAF as a candidate for the regulation of IL-10 production in human Th17 cells, thus potentially representing a discriminant factor between pathogenic and non pathogenic Th17 cells.

Via Vincenzo Vela 6
6500 Bellinzona, Switzerland
Tel. +41 91 820 0300
Fax +41 91 820 0302
Facebook / Twitter
